A00-280 Online Practice Questions and Answers
The following question will ask you to provide a line of missing code. Given the following data set work.vs:

The following SAS program is submitted to create a new data set that carries forward the previous value of sbp when the value is missing.
In the space below, enter the line of code that completes the program (Case is ignored. Do not add leading or trailing spaces to your answer.).
This question will ask you to provide a line of missing code. The following SAS program is submitted:

In the space below, enter the statement that completes the program correctly (Case is ignored. Do not add leading or trailing spaces to your answer.).
Which CDISC filename contains the following items?
Variable attributes
Controlled terminology
Computational methods
Enter your answer in the space below (Case is ignored. Do not add leading or trailing spaces to your answer.).
The following SAS program is submitted:
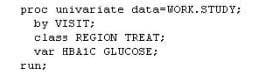
You want to store all calculated means and standard deviations in one SAS data set.
Which statement must be added to the program?
A. output mean std;
B. ods output mean=m1 m2 std=s1 s2;
C. output out=WORK.RESULTS mean=m1 m2 std=s1 s2;
D. ods output out=WORK.RESULTS mean=m1 m2 std=s1 s2;
This question will ask you to provide a line of missing code.
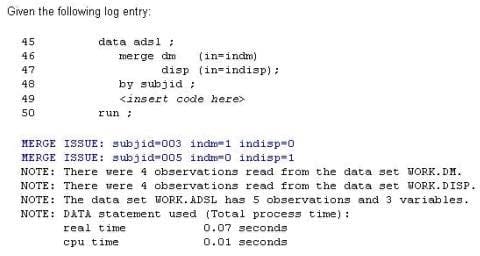
Which line of code would produce the blue notes in the log?
A. if indm ne indisp then output 'MERGE ISSUE: ' subjid indm indisp ;
B. if indm ne indisp then put 'MERGE ISSUE: ' subjid= indm= indisp=;
C. %if indm ne indisp %then %put 'MERGE ISSUE: ' subjid= indm= indisp=;
D. if indm ne indisp then put 'MERGE ISSUE: ' _all_ ;
Given the following data set DEMOG:
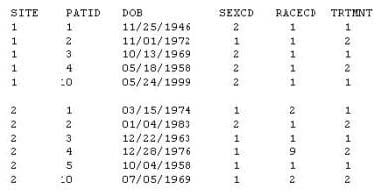
Which selection below would be considered hard-coding?
A. if sexcd eq 1 then sex = "Male" ; else if sexcd eq 2 then sex = "Female" ;
B. if site eq 1 then sexcd = 2 ; else if site eq 2 then sexcd = 1 ;
C. if site eq 1 and sexcd ne 2 then check = 1 ; else if site eq 2 and sexcd ne 1 then check = 2 ;
D. birthdt = input(dob, mmddyy10.) ;
Given the following data set:
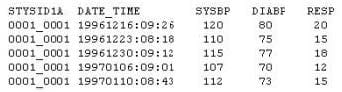
Which type of clinical trials data is this?
A. Demographics
B. Laboratory
C. Medical History
D. Vital Signs
A subject reports a medication started in March of 2007 but cannot recall the day number. What is the value stored in the SDTM domain CM.CMSTDTC variable?
A. 00MAR2007
B. 2007 03
C. MAR2007
D. 2007-03
